سدیم سولفات انهیدروس
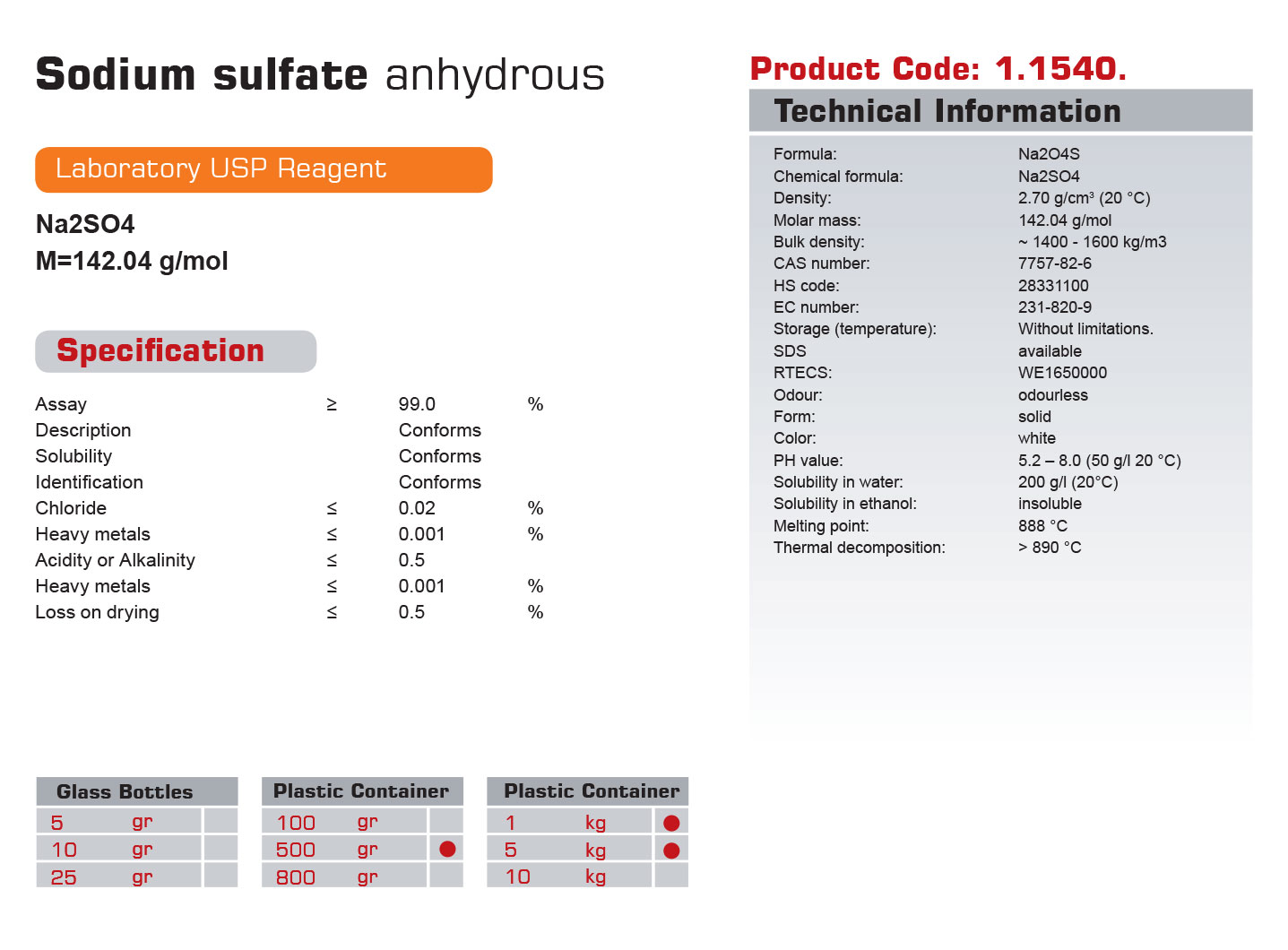
| Formula: | Na2O4S |
| Chemical formula: | Na2SO4 |
| Density: | 2.70 g/cm3 (20 °C) |
| Molar mass: | 142.04 g/mol |
| Bulk density: | ~ 1400 – 1600 kg/m3 |
| CAS number: | 7757-82-6 |
| HS code: | 28331100 |
| EC number: | 231-820-9 |
| Storage : | Without limitations. |
| SDS | available |
| RTECS: | WE1650000 |
| Odour: | odourless |
| Form: | solid |
| Color: | white |
| PH value: | 5.2 – 8.0 (50 g/l 20 °C) |
| Solubility in water: | 200 g/l (20°C) |
| Solubility in ethanol: | insoluble |
| Melting point: | 888 °C |
| Thermal decomposition: | > 890 °C |
| Assay | ≥ | 99 | % |
| Description | Conforms | ||
| Solubility | Conforms | ||
| Identification | Conforms | ||
| Chloride | ≤ | 0/2 | % |
| Heavy metals | ≤ | 0/001 | % |
| Acidity or Alkalinity | ≤ | 0/5 | % |
| Heavy metals | ≤ | 0/001 | % |
| Loss on drying | ≤ | 0/5 | % |
Sodium sulfate anhydrous is a white, crystalline inorganic salt widely used in pharmaceutical laboratories, chemical manufacturing, and analytical chemistry, particularly known for its role as a drying agent and a reagent in various chemical reactions.
🏭⚗️ Production
Sodium sulfate anhydrous is commonly produced as a byproduct in industrial processes such as the manufacture of hydrochloric acid from sodium chloride and sulfuric acid, or during the production of sodium dichromate. It can also be obtained by dehydrating naturally occurring Glauber’s salt (Na₂SO₄·10H₂O) through heating, which removes water of crystallization and yields the anhydrous form.
🔬 Properties
The chemical formula is Na₂SO₄, with a molar mass of approximately 142.04 g/mol. Sodium sulfate anhydrous appears as a white, odorless crystalline powder that is highly soluble in water and insoluble in ethanol. It has a melting point of approximately 884 °C and a density of about 2.66 g/cm³. It is chemically stable, non-hygroscopic in its anhydrous form, and acts as a neutral salt in solution. Its high thermal stability makes it suitable for high-temperature applications in chemical reactions.
🧪 Applications
In laboratory and pharmaceutical contexts, sodium sulfate anhydrous is widely used as a drying agent for removing water from organic solvents due to its strong affinity for moisture. It is also used in quantitative chemical analysis, sample preparation, and precipitation reactions. In the pharmaceutical industry, it has been utilized in the preparation of certain formulations and as a controlled-release matrix material. Additionally, it is used in dyeing processes, paper manufacturing, and in the synthesis of other sodium-based compounds.
⚠️ Safety
Sodium sulfate anhydrous is generally considered non-toxic and environmentally safe under normal handling conditions. However, excessive inhalation of dust may cause minor respiratory irritation, and prolonged skin contact can lead to dryness. While it poses minimal chemical hazards, standard laboratory safety protocols should be followed during handling, including the use of gloves, lab coats, and dust masks if necessary. It should be stored in a dry, sealed container to prevent moisture uptake and caking.