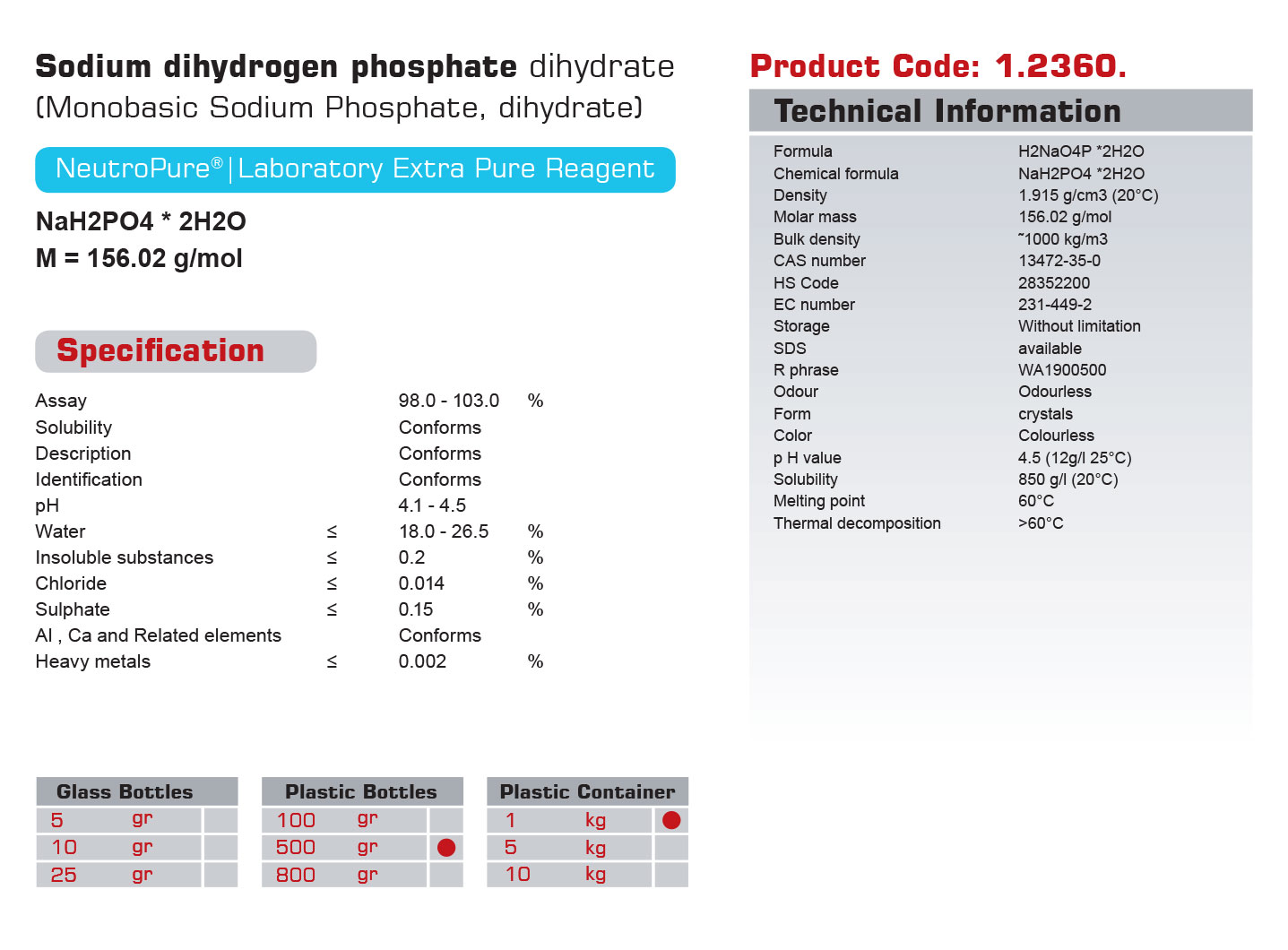
سدیم دی هیدروژن فسفات دی هیدرات
| Chemical formula | H2NaO4P*2H2O |
| Molar mass | 156.02 g/cm3 |
| CAS Number | 13472-35-0 |
| HS Code | 28352200 |
| Storage | With out limitation |
| EC number | 231-449-2 |
| SDS | available |
| Odour | odourless |
| Form | crystals |
| Color | colorless |
| pH value | 4/5 |
| Solubility in water | 850 g/l |
| Melting point | 60°C |
| Thermal decomposition | 60 °C |
| Description | Conforms | ||
| Identification | Conforms | ||
| Solublity | Conforms | ||
| Insoluble substances | ≤ | 0/2 | % |
| Chloride | ≤ | 0/014 | % |
| Sulphate | ≤ | 0/15 | % |
| Heavy metals | ≤ | 0/002 | % |
| Aluminium ,Calcium & related elements | ≤ | Conforms | |
| Arsenic | ≤ | 8 | ppm |
| Iron | ≤ | 0/003 | % |
| pH | 5.1 to 4.5 | ||
| Sulfated ash | ≤ | 0/15 | % |
| Water | 18.0 to 26.5 | % | |
| Assay | 98.0 to 103.0 | % |
Sodium dihydrogen phosphate dihydrate is the dihydrated form of sodium dihydrogen phosphate, an inorganic compound with the chemical formula NaH₂PO₄·2H₂O. It is a white, crystalline solid commonly used in food, water treatment, and as a buffering agent in laboratories.
🏭⚗️ Production
Sodium dihydrogen phosphate is produced by the neutralization of phosphoric acid (H₃PO₄) with sodium carbonate (Na₂CO₃) or sodium hydroxide (NaOH), followed by controlled crystallization to obtain the dihydrate form.
🔬 Properties
It is a white, odorless, water-soluble solid. The dihydrate form melts around 60 °C (with loss of water). In aqueous solutions, it is mildly acidic (pH ~4.5–5 when dissolved in water), making it useful in buffer systems. It is stable under normal conditions and non-combustible.
🧪 Applications
• Buffering agent: Used in biological and chemical laboratories to maintain stable pH in phosphate buffer systems.
• Food additive (E339): Acts as an emulsifier, acidity regulator, and nutrient supplement.
• Water treatment: Helps prevent corrosion in pipes and scale formation.
• Pharmaceuticals: Used in some oral and intravenous formulations as a pH adjuster.
• Agriculture: Sometimes included in fertilizer formulations as a phosphorus source.