دی کلرومتان (متیلن کلرید)
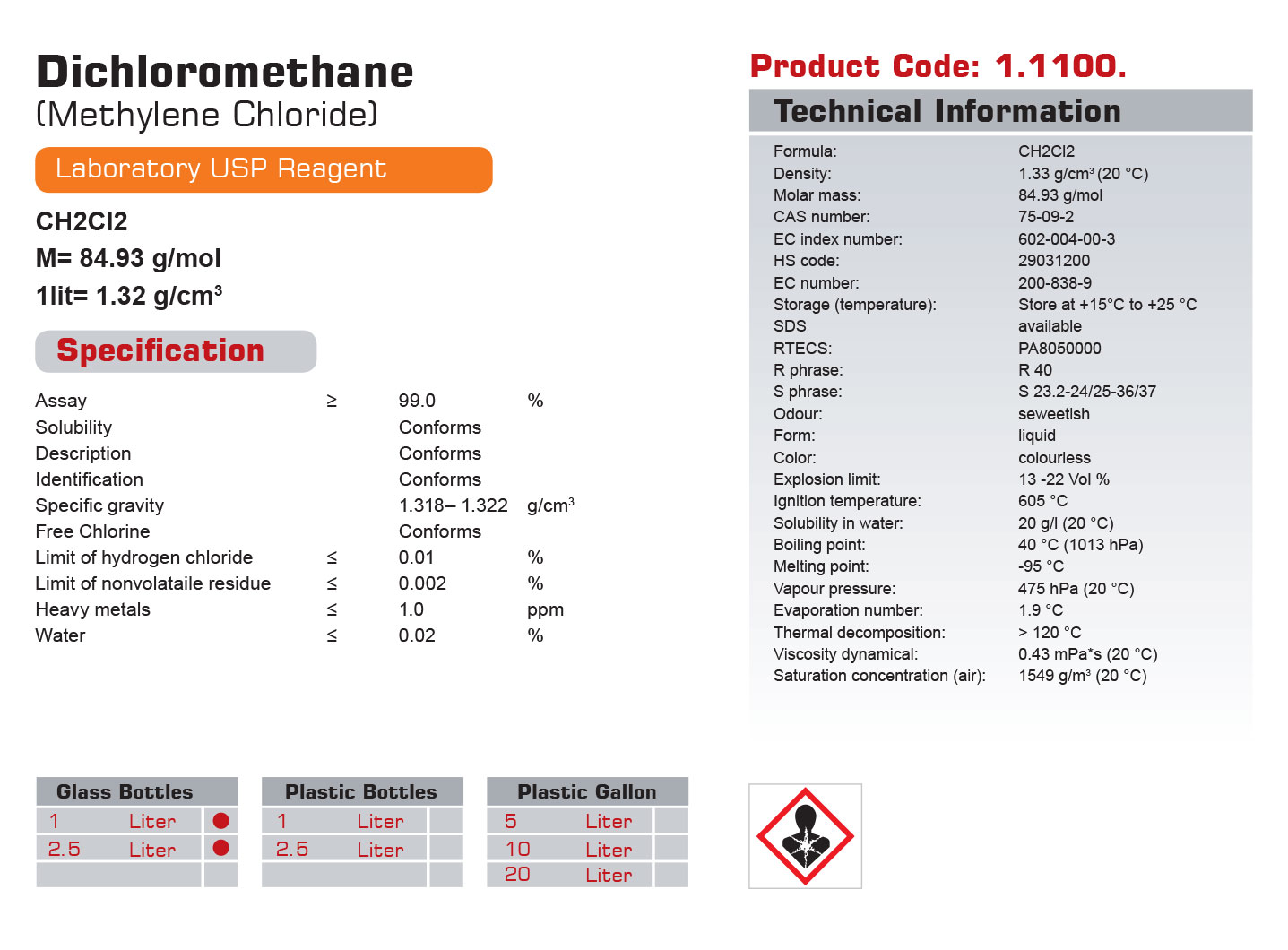
| Formula: | CH2Cl2 |
| Density: | 1.33 g/cm3 (20 °C) |
| Molar mass: | 84.93 g/mol |
| CAS number: | 75-09-2 |
| EC index number: | 602-004-00-3 |
| HS code: | 29031200 |
| EC number: | 200-838-9 |
| Storage (temperature): | Store at +15°C to +25 °C |
| SDS | available |
| RTECS: | PA8050000 |
| R phrase: | R 40 |
| S phrase: | S 23.2-24/25-36/37 |
| Odour: | seweetish |
| Form: | liquid |
| Color: | colourless |
| Explosion limit: | 13 -22 Vol % |
| Ignition temperature: | 605 °C |
| Solubility in water: | 20 g/l (20 °C) |
| Boiling point: | 40 °C (1013 hPa) |
| Melting point: | -95 °C |
| Vapour pressure: | 475 hPa (20 °C) |
| Evaporation number: | 1.9 °C |
| Thermal decomposition: | > 120 °C |
| Viscosity dynamical: | 0.43 mPa*s (20 °C) |
| Saturation concentration | 1549 g/m3 (20 °C) |
| Assay | ≥ | 99.0 | % |
| Solubility | Conforms | ||
| Description | Conforms | ||
| Identification | Conforms | ||
| Specific gravity | 1.318– 1.322 | g/cm3 | |
| Free Chlorine | Conforms | ||
| Limit of hydrogen chloride | ≤ | 0.01 | % |
| Limit of nonvolataile residue | ≤ | 0.002 | % |
| Heavy metals | ≤ | 1.0 | ppm |
| Water | ≤ | 0.02 | % |
Dichloromethane (Methylene chloride) is an organochlorine compound with the chemical formula CH₂Cl₂. It is a colorless, volatile liquid with a mildly sweet, chloroform-like odor. Though only slightly soluble in water, it dissolves readily in many organic solvents and is widely used as an industrial and laboratory solvent.
🏭⚗️ Production
Dichloromethane is produced industrially by the chlorination of methane at high temperatures. This reaction yields a mixture of chlorinated methanes, including chloromethane, dichloromethane, chloroform, and carbon tetrachloride. These compounds are then separated through distillation.
🔬 Properties
Dichloromethane is a dense, non-flammable, colorless liquid with a boiling point of about 39.6 °C and a melting point of –96.7 °C. It has a density of approximately 1.33 g/cm³ at room temperature. It evaporates quickly and is heavier than water. While it is only slightly soluble in water, it is miscible with many organic solvents including alcohols, ethers, and ketones. It is relatively stable but can decompose at high temperatures to produce toxic gases such as phosgene and hydrogen chloride.
🧪 Applications
• Solvent: Widely used in paint removers, degreasers, adhesives, and coatings
• Pharmaceuticals: Used as a solvent in drug manufacturing and capsule production
• Food industry: Formerly used in decaffeinating coffee and tea (now restricted in many countries)
• Plastics: Employed in bonding and welding of plastic parts
• Laboratory: Commonly used as an extraction solvent in chemical and biological labs