Sodium hydroxide solution 50%
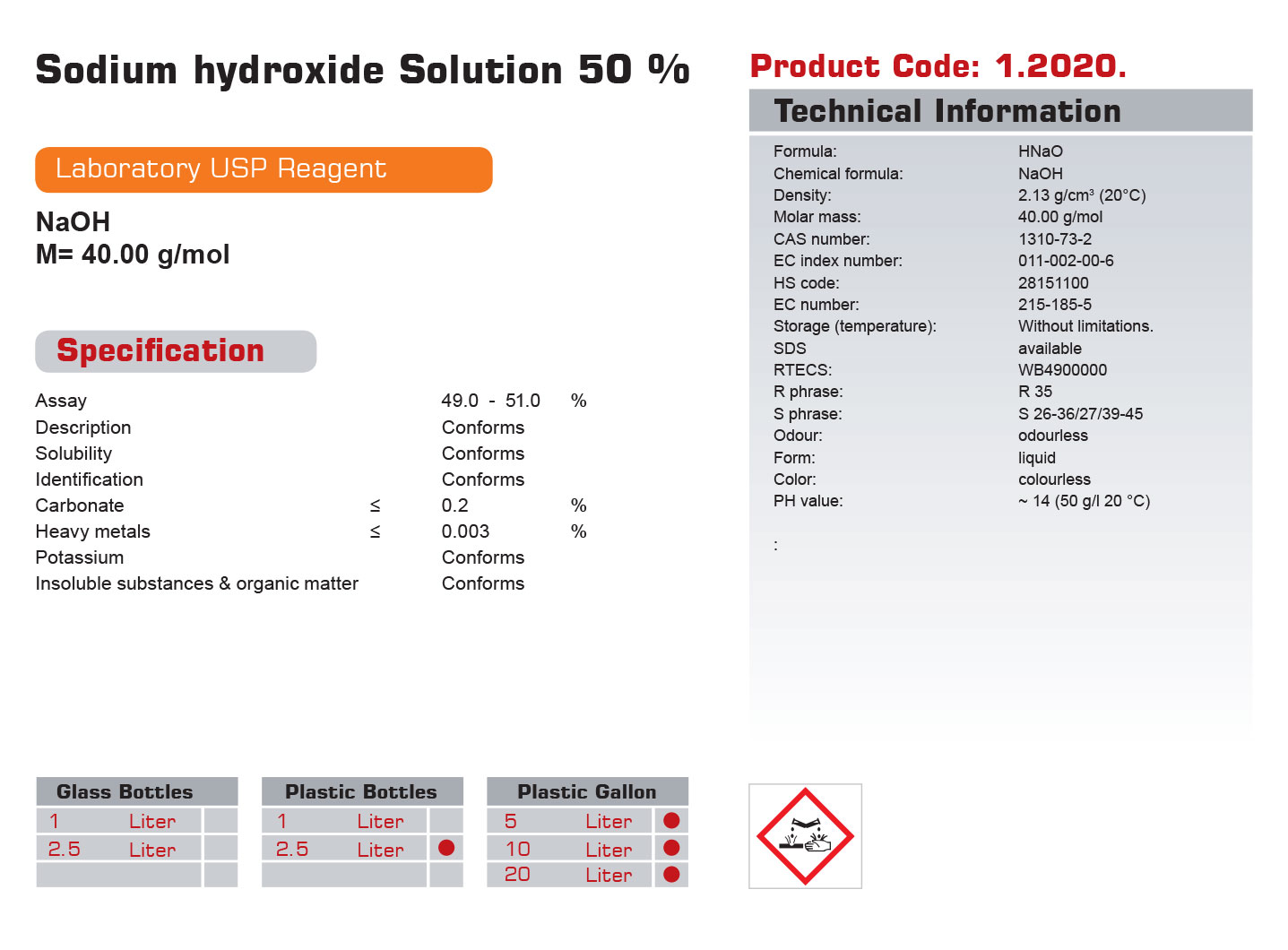
| Formula: | HNaO |
| Chemical formula: | NaOH |
| Density: | 2.13 g/cm3 (20°C) |
| Molar mass: | 40.00 g/mol |
| CAS number: | 1310-73-2 |
| EC index number: | 011-002-00-6 |
| HS code: | 28151100 |
| EC number: | 215-185-5 |
| Storage (temperature): | Without limitations. |
| SDS | available |
| RTECS: | WB4900000 |
| R phrase: | R 35 |
| S phrase: | S 26-36/27/39-45 |
| Odour: | odourless |
| Form: | liquid |
| Color: | colourless |
| PH value: | ~ 14 (50 g/l 20 °C) |
| Assay | 53.0-57.0 | % | |
| Description | Conforms | ||
| Solubility | Conforms | ||
| Identification | Conforms | ||
| Carbonate | ≤ | 0.2 | % |
| Heavy metals | ≤ | 0.003 | % |
| Potassium | Conforms | ||
| Insoluble substances & organic matter | Conforms |
Sodium hydroxide solution 50% is an aqueous solution of sodium hydroxide (NaOH), commonly known as caustic soda or lye. At this concentration, it is a clear, colorless, highly caustic liquid widely used in chemical manufacturing, water treatment, and industrial cleaning.
🏭⚗️ Production
Sodium hydroxide solution is typically produced by the chloralkali process, in which electrolysis of a sodium chloride (NaCl) solution yields chlorine gas, hydrogen gas, and sodium hydroxide. The resulting NaOH is often concentrated to 50% by evaporation for industrial use.
🔬 Properties
A 50% NaOH solution is highly alkaline and corrosive, with a high pH (~14). It is hygroscopic, absorbs carbon dioxide from air, and reacts exothermically with acids and water. The solution is stable under normal conditions but must be stored in corrosion-resistant containers.
🧪 Applications
• Chemical industry: Used in the manufacture of paper, textiles, detergents, and soaps.
• Water treatment: Used to adjust pH and neutralize acidic waste streams.
• Food industry: Used in regulated amounts for cleaning equipment and peeling fruits/vegetables.
• Pharmaceuticals: Used in pH adjustment and cleaning processes.
• Industrial cleaning: Acts as a powerful degreaser and drain cleaner.