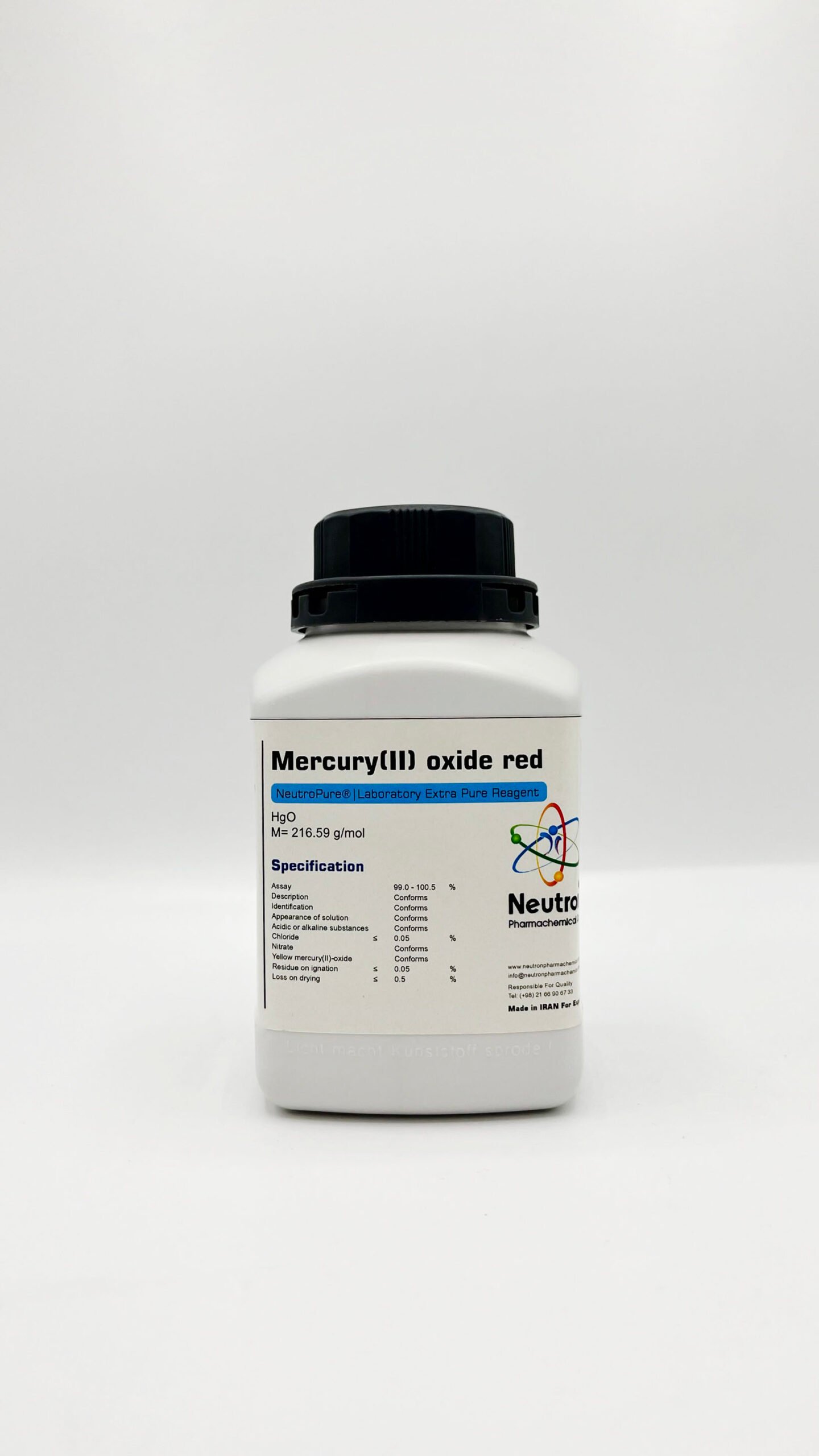
Mercury (ii) oxide red
| Chemical formula | HgO |
| Density | 11.1 g/cm3 (20°C) |
| Molar mass | 216.59 g/mol |
| Bulk density | ~3000 kg/m3 |
| CAS number | 21908-53-2 |
| HS Code | 28259050 |
| EC number | 244-654-7 |
| Storage | Without limitation |
| SDS | available |
| RTECS | OW8750000 |
| R phrase | R 26/27/28-33-50 |
| S phrase | S 13-28.1-45-60 |
| Odour | odourless |
| Form | solid |
| Color | red |
| Assay | 99.0 – 100.5 | % | |
| Description | Conforms | ||
| Identification | Conforms | ||
| Appearance of solution | Conforms | ||
| Acidic or alkaline substances | Conforms | ||
| Chloride | ≤ | 0.05 | % |
| Nitrate | Conforms | ||
| Yellow mercury(II)-oxide | Conforms | ||
| Residue on ignation | ≤ | 0.05 | % |
| Loss on drying | ≤ | 0.5 | % |
Mercury(II) oxide red is a bright red crystalline inorganic compound primarily used in laboratory settings and in the production of elemental mercury through thermal decomposition.
🏭⚗️ Production
It is typically prepared by heating elemental mercury in the presence of oxygen at elevated temperatures or by thermal decomposition of mercury(II) nitrate, followed by controlled cooling to obtain the red crystalline form.
🔬 Properties
The chemical formula is HgO with a molar mass of approximately 216.59 g/mol. It appears as a red or orange-red powder, is practically insoluble in water, and decomposes upon heating to release oxygen and metallic mercury. The red form is thermodynamically more stable than the yellow form and is sensitive to light and heat.
🧪 Applications
Mercury(II) oxide red is used in the preparation of pure mercury, in analytical chemistry, and in historical applications such as in batteries and antiseptics. It has also been used as a reagent in organic synthesis, though its use has declined due to toxicity concerns.
⚠️ Safety
This compound is highly toxic and hazardous to health and the environment. It is dangerous if inhaled, ingested, or upon skin contact, and can cause damage to the kidneys and central nervous system. Decomposition releases toxic mercury vapor. Proper protective equipment such as gloves, masks, and eye protection is essential, and it must be handled in a well-ventilated area or fume hood and stored in tightly sealed containers away from light and heat.