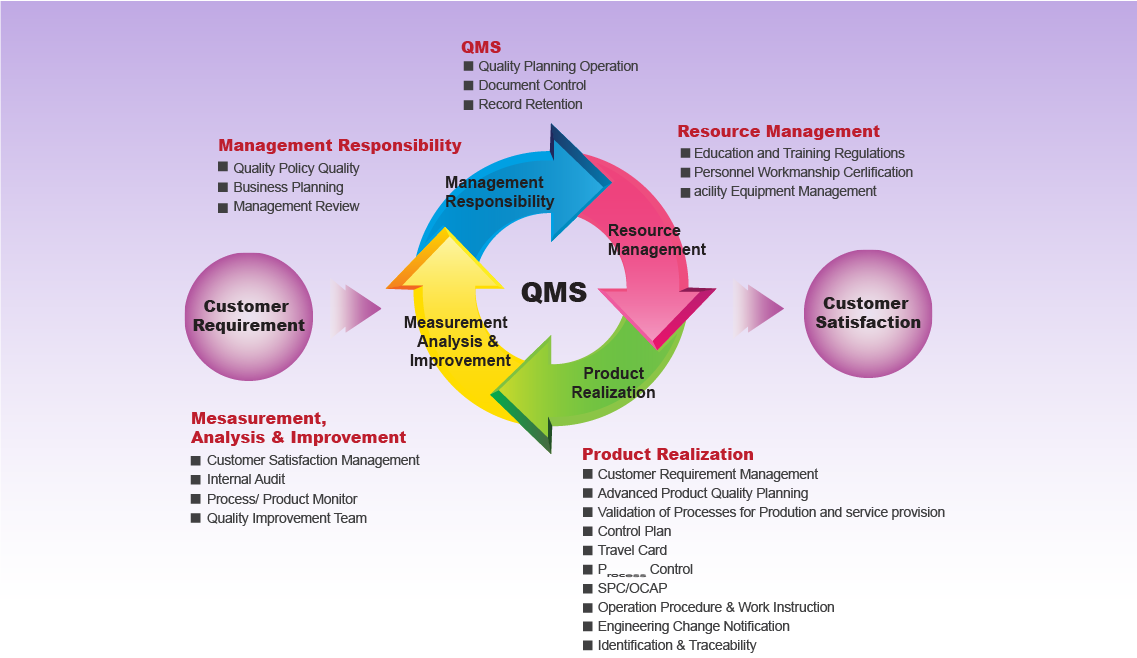
As one of the most specialized manufacturers of laboratory materials and reagents, chemical, biochemical, medical diagnosis, pathobiology, disinfectants, pharmaceutical auxiliary materials, dental materials and calibration solutions with optimal quality in Iran, Neutron Pharmaceutical Chemical Company always tries to take great steps towards implementation and commitment. to quality management systems, improving the quality of products, developing activities and services in order to maintain the dignity and maintenance of customers, beneficiaries and human resources, and also considers itself committed to fulfilling the legal requirements and requirements of the General Administration of Medical Equipment and Pharmaceutical Raw Materials.
Therefore, in this regard, he has put the following policies at the top of his work:
1. Efforts to increase customer satisfaction
2. Developing the product portfolio and increasing production efficiency
3. Improving the skills of personnel in order to improve the quality of products
4. Establishing safe environmental conditions for employees
5. Protecting the environment by preventing adverse environmental consequences from activities
In order to fulfill this policy, Neutron Pharmaceutical Chemical Company quality management systems
ISO13485:2016, ISO 9001:2015, ISO14001:2015, ISO45001:2018 and GMP have been implemented in the organization and according to the policies defined above, it has defined quality goals and prepared operational plans to achieve these goals. Risk-based approach is one of the principles of this company in carrying out all activities, especially those affecting product quality.
